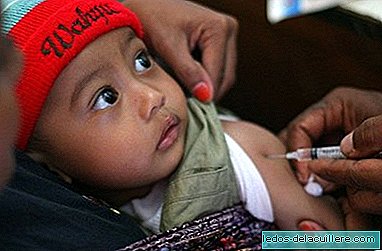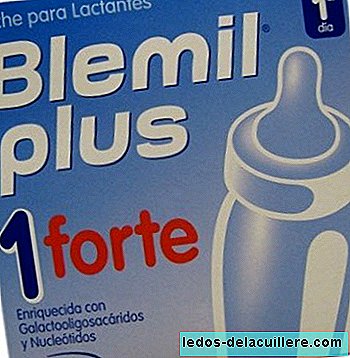
We publish today the second part of the long interview given to us by Montserrat Rivero, General Scientific Director of Ordesa Laboratories, motivated by the social concern caused by the withdrawal of a batch of Blemil Plus 1 and the subsequent news of an outbreak of salmonella that, without being demonstrated, was initially reported to be possibly caused by this milk, although as of today There is no official confirmation.
We wanted to know the data available to the company about the possible relationship between both circumstances and also, their contacts with the affected families, expanding with this second installment the information we already gave in the first part of this interview. We have also asked the questions that, as parents, we believe may be useful to consumers of this product.
Have you taken any steps to contact the affected families?
Of course. Our main interest has been to care for all babies who have been sick, regardless of the type of salmonella they had or what milk they had taken. We have spoken, with the majority on more than one occasion, with all those who have contacted us. We could not do it with those of whom we did not have your data. To facilitate the contact we put at your disposal a telephone and an email from the first moment of the withdrawal of the affected lot. Our only objective was to put ourselves at your disposal in order to provide all the necessary support. The company keeps the telephone number 902.105.243 and the email address [email protected] available to the public to answer any questions that may arise.I want to insist on pointing out that, as a company dedicated to infant feeding and as people, the natural reaction is to show solidarity with any family that suffers for the health of their children, whatever the problem they may suffer. I am a pharmacist and scientist myself, but first I am a mother and grandmother. Currently, we continue to contact some of the affected families to follow the evolution of their babies.
Was the information given to the public about the collection of the containers by the health authorities correct? What protocol was followed exactly?
As a company we inform you, as soon as you withdraw the lot, from the national and regional health authorities, as well as from the Official Pharmaceutical Associations, which, according to the established legal framework, are the official bodies in charge of the timely communication of the withdrawal of an affected lot of product to Pharmacies I do not know what is the protocol of action that the health authorities applied from that moment and on which they based their decisions.
What is the reason that the problem lot was indicated as a possible cause if this fact was not confirmed? Is it possible that the contamination came from this lot even though it is not confirmed analytically?
Let me not speculate on a subject as delicate as this. What I can tell you is that, every year, there are about 200 cases of salmonella in infants under one year old in Spain due to viral or bacterial infections.In the case of lot 236, I strongly believe that the only way to establish a direct cause-effect relationship is precisely the confirmation of analytics following the official procedure.
Have your sales dropped since then?
Believe me, sales are not what matters now. The fundamental thing is to transmit to the mothers, so that they are calm, that the problem of tightness occurred in a specific lot and not in the whole range and that we are a responsible and committed company with the consumer. We are not a multinational, as many people think, but a Spanish company with a vocation for service that, in a timely manner, has been associated with a problem that some families that consumed one of their products are suffering without there being any evidence to show that Milk is the origin of your problem. We are mainly concerned with these families and their children but also with the rest of the people who trust us: customers, pharmacists, suppliers and employees.
How will the company transmit a message of confidence to users after what happened?
First, telling them that we are a reference company in our sector backed by 60 years of experience, which researches and offers quality products. The lack of tightness has been something unpredictable and specific to a few cans that had never happened before. Finally, insist that we work on continuous improvement. Secondly, to point out that for us the well-being of our consumers is fundamental and that for this we have hygienic-sanitary and traceability control systems with very high levels of safety.
Have there been other cases of contamination or preventive withdrawals in the history of the company or of the packaging plant?
In 2007 the company was forced by the Health Authorities to withdraw the Blevit Digest infusion which was manufactured in Switzerland. It was a precipitous and inconsistent measure and I rely on this statement that finally the Health Authorities concluded, at the end of their investigation, that Blevit Digest had no relation to possible cases of contamination. This measure caused serious damage to the reputation of the product that until then had been very useful for many families.
Do you think that the quality control protocols required for this type of products are sufficient?
Yes, it should be borne in mind that quality controls for baby food are very rigorous and are determined by European directives endorsed by renowned specialists and associations. In the case of Ordesa, internal controls always exceed the established legal standards since we carry out 430 analyzes in a batch, both physically and chemically and microbiologically.
Do you want to send a message to families who have children who have been sick and consumed the milk in this batch?
We want to tell you the same thing that we have personally said to the people who have contacted us: that we understand their anguish and concern and that we are at their side if we can help them with anything. But we also want to ask you not to get carried away by little rigorous information or unsubstantiated comments that only cause alarm. For example, it is not true that we are the same company that manufactures Novalac nor that salmonella is reproduced by eggs. If there has been a problem with lot 236 0, it could have been due to the loss of tightness of specific cans as far as we know so far, but we neither lied nor have been negligent. We would also like to remind parents that we removed the lot preventively and that our attitude was responsible. You can always say that we could do more, but believe me when I tell you that we did inform the consumer, by various means, and that if we did not do it massively it was because we were convinced that there was no longer a product in the market.Finally, we would like to tell any family that has a sick child and has consumed that lot, to call us so that we can contrast the information, answer their questions and help them in whatever is in our hands.
With this we finish the interview with Montserrat Rivero, General Scientific Director of Ordesa Laboratories, which we hope will help consumers and those affected by the salmonella outbreak, have at their disposal all the necessary information and we commit ourselves to follow this case to be able to offer our readers the news that happens, if any, from From now.